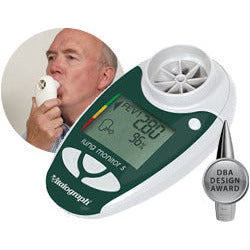
Vitalograph Lung Monitor USB Respiratory Monitor
Vitalograph Lung Monitor USB Respiratory Monitor
£193.99 ex.VAT
£193.99 ex.VAT
AVAILABLE TO BACKORDER - Buy now for despatch when stock arrives.
Couldn't load pickup availability
SKU: 40700
More like this
More like this
Shipping
Shipping
FREE STANDARD SHIPPING available on all orders over £25 EX VAT.
Next working day - available until 8pm Monday to Friday
Weekend despatch - available until 12pm Sunday for MONDAY delivery (Next working day)
Sat and Sunday delivery service - available until 5pm Friday.
Why buy from Medisave?
Why buy from Medisave?
Medisave supplies health professionals, business and home users.
- Credit card, PayPal and Amazon Payments accepted
- Free delivery over £25 ex VAT
- NHS, school or company purchase order - email accounts@medisave.co.uk
- Laser engraving
- Delivery to private or commercial addresses
- Google Certified Shop
- Registered with the Medicines and Healthcare products Regulatory Agency (MHRA)
- ISO9001 quality management system + ISO 140001 environmental
- Established 1999
Need some advice? Speak to our friendly team on 0800 804 6447
Share
For the home monitoring of FEV1, FEV6 & the ratio with electronic & hard copy reports
The lung monitor USB combines all of the features of the lung monitor with the ability to link to your PC. Session data and device ID may be transmitted to Vitalograph Reports or an API developers' kit is available for providers of e-Diary, home hub and telemedicine solutions.
- BMI calculated
- Blow quality and number of blows indicated
- Ability to add comments to the report
- Information presented in tabular and graphical form
- Report headers can be customised
- Automatically names the pdf file using subject name and date
Reports include:
- Subject ID and name
- Age, height, gender, and weight fields optional
- Best FEV1, FEV6 and ratio automatically populated
- Personal best FEV1 set in the lung monitor
- Device ID and time/date fields
- Automatically populated



![FFP3 Face Mask x 10 [Valved]](http://cdn.shopify.com/s/files/1/0715/4896/1051/products/img_2280_1_540x.jpg?v=1679930259)